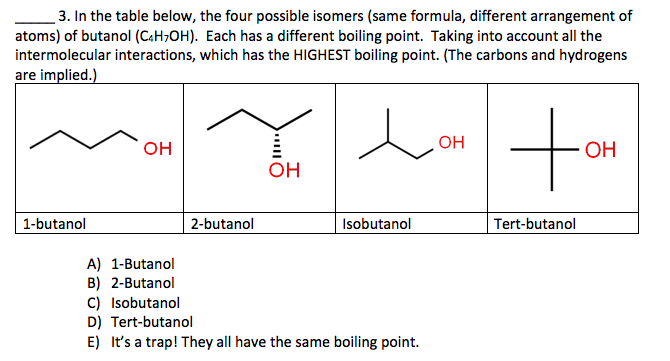
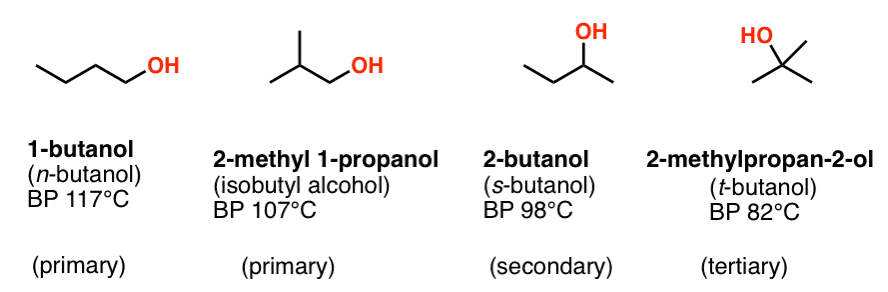
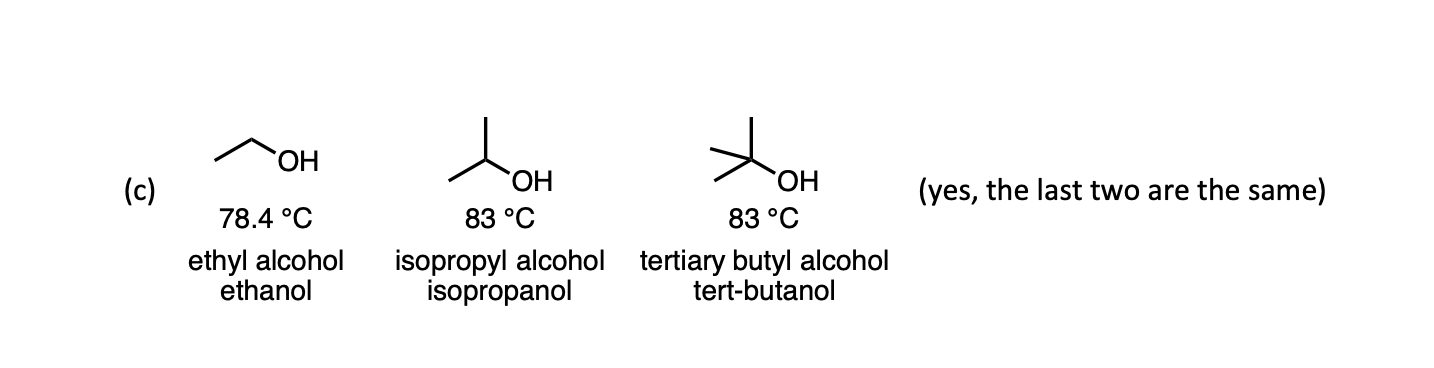
SOLVED: Measure the bp of each ofthe butyl alcohol isomers listed below: (Note you already have result for n-butyl alcohol from part A Note that all these structural isomers have identical molar
Correct statement(s) in case of n-butanol and t-butanol is (are):A. both are having equal solubility in water.B. t-butanol is more soluble in water than n-butanol.C. The boiling point of t-butanol is lower

Arrange the following compounds in order of their increasing boiling points.n - butyl alcohol, glycerol, n - butane, tert - butyl alcohol, sorbitol, n - butyraldehyde, isobutyl alcohol.

Tert-Butanol, Certified Reference Material, MilliporeSigma Supelco 20 mL:Chemicals, | Fisher Scientific